Biomarkers for Tuberculosis
Tuberculosis (TB) remains one of the leading causes of mortality worldwide, driven largely by delayed diagnosis and the emergence of drug-resistant strains. In recent years, the development and validation of biomarkers for tuberculosis have become central to improving early detection, disease monitoring, and therapy efficacy. Whether in the context of latent TB infection (LTBI) or active pulmonary TB, identifying reliable and reproducible biomarkers is a top priority in both clinical and research settings.
Biomarkers for TB Consortium is part of the Grand Challenges in Global Health initiative which was launched in 2003 by the Bill & Melinda Gates Foundation. In partnership with the National Institutes of Health, Wellcome Trust and Canadian Institute of Health to harness the power of science and technology to dramatically improve health in the world’s poorest countries.
The Grand Challenges initiative is supporting groundbreaking research projects to discover and develop scientific breakthroughs for preventing, treating, and curing diseases that kill millions of people each year in developing countries.
A grand challenge is a call for a specific scientific or technological innovation that would remove a critical barrier to solving an important health problem in the developing world with a high likelihood of global impact and feasibility. A grand challenge is neither the statement of the global health problem itself (e.g., malaria or AIDS) nor the request for a specific health intervention (e.g., a drug or vaccine), but the call for a discrete scientific or technological innovation which will break through the roadblock that stands between where we are now and where we would like to be in science, medicine, and public health. The grand challenges are goal oriented under 7 goals encompassing thematic topics. A total of 14 Grand Challenges were identified through a call for submissions from more than 1,000 scientists and public health leaders around the world.

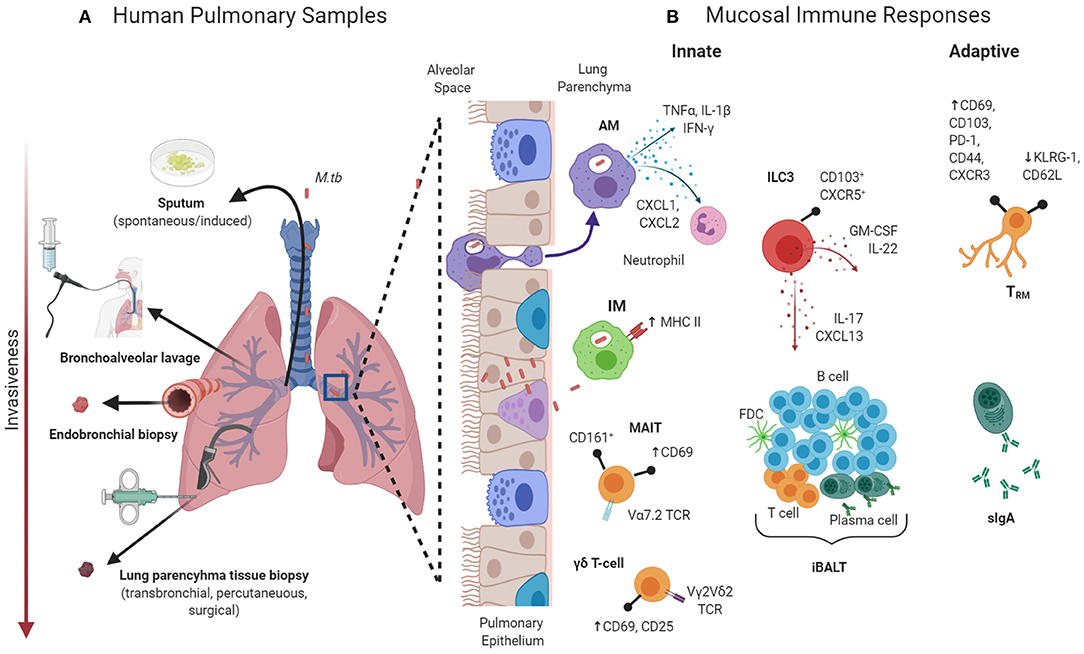
Why Are Biomarkers Important in TB Research?
Biomarkers offer a non-invasive and precise method for:
- Differentiating latent from active TB
- Monitoring treatment response
- Predicting disease progression
- Enabling point-of-care testing in resource-limited areas
To support these efforts, a wide range of molecular biology tools and reagents are employed in biomarker research.
Biomarkers of Protective Immunity Against TB in the Context of HIV/AIDS in Africa.
Dr. Kaufmann will lead an international consortium of 15 institutions in Europe, Africa, and the U.S. They will attempt to identify immune system differences between people who are exposed to tuberculosis and never become sick, and those who develop serious disease. The researchers will focus particular attention on people infected with both TB and HIV. The study results could help guide the design and testing of new TB vaccines, drugs, and diagnostics, especially in areas with high HIV infection rates.

1. Host-Derived Biomarkers
Host immune response is key in TB. Cytokines, such as IFN-γ, TNF-α, and IL-2, along with markers like C-telopeptide and acetylated p53 antibody, are studied for their diagnostic relevance. Blood-based markers using exosome ELISA kits, especially from exosome-depleted FBS samples, allow for profiling of immune signaling molecules.Techniques such as western blot using positive controls, ATP measurement kits, and DAPI mounting medium help visualize and quantify protein-level changes in TB progression.

2. Bacterial Antigens and Metabolites
Surface antigens from Mycobacterium tuberculosis—like ESAT-6, CFP-10, and 38kDa protein—are powerful TB biomarkers. Additionally, TBARS assay kits are used to measure oxidative stress, often elevated during infection. For serological testing, lateral flow assay kits are widely employed in point-of-care settings.The use of microtubes, Seahorse ATP kits, and lateral flow platforms are essential for validating these biomarkers under standardized conditions like 0.5 McFarland standards.
Sample Processing and Tools in TB Biomarker Discovery
Successful biomarker discovery relies heavily on sample quality and processing methods. The following tools play a vital role in standardizing TB research:
- Lympholyte M and SepMate™ tubes : Efficient PBMC isolation
- CSF collection tubes: For diagnosing TB meningitis
- PowerSoil DNA Isolation Kits : Ideal for microbiome-associated TB studies
- Tumor dissociation kits (human): Repurposed for tissue disaggregation in granuloma studies
- Instant Blue: Rapid protein staining in gel electrophoresis workflows
- Seamless cloning techniques for recombinant antigen production
- Banana lectin and Griffonia simplicifolia: Glycan-binding proteins useful in TB host-pathogen interaction studies

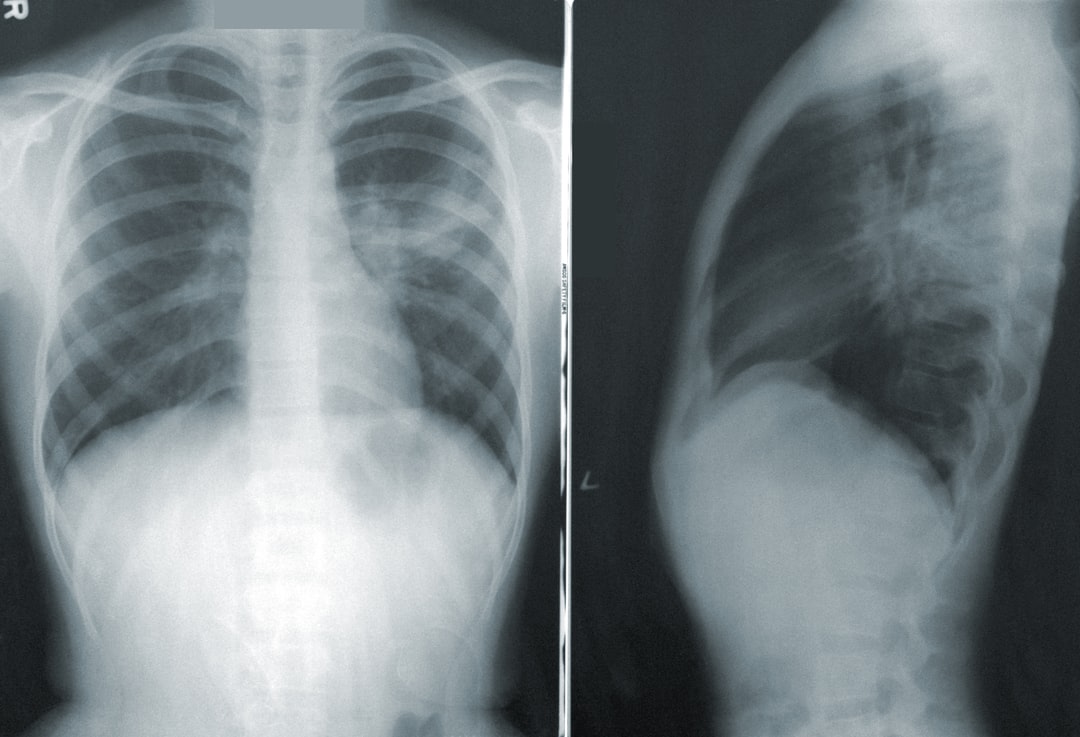
Background of problem – Tuberculosis
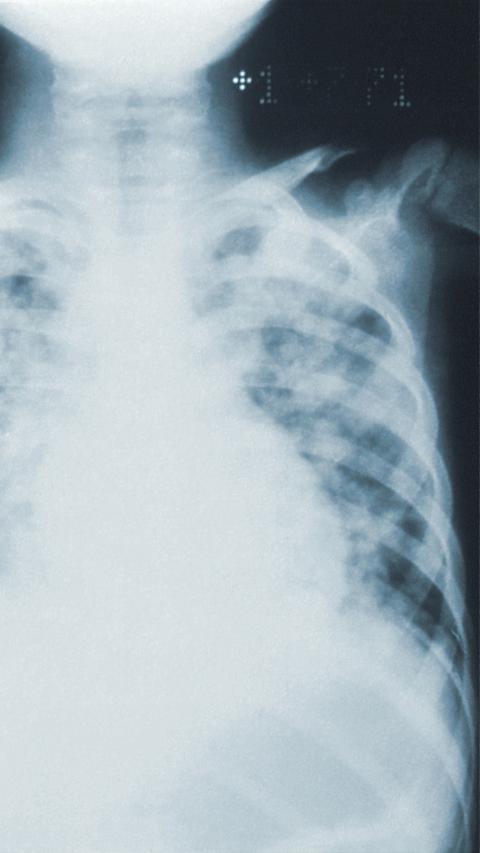
TB is a major health problem especially in low income countries disproportionally affecting the developing world. Each year it is estimated that more than 8 million people develop TB with over 2 million deaths. Based on tuberculin skin-test surveys it is estimated that one third of the global population is already latently infected with Mycobacterium tuberculosis (Mtb). Typically, only 1 in 10 infected individuals will develop TB disease during lifetime, but with progressive HIV-1 coinfection the risk increases about 8% per year. In Africa, HIV-1 has been the single most important factor in determining the increased incidence of TB in the past 10 years.
Despite major efforts to implement effective treatment strategies for TB patients and the widespread use of the vaccine Bacille Calmette-Guérin (BCG) in infants, none of the current TB control measures appear to address the issue of the enormous reservoir of latent TB that keeps perpetuating the epidemic. From a public health point of view the development of novel TB interventions, in particular post-exposure vaccines that will eradicate latent TB, are expected to be very cost-effective in global TB control and will complement current treatment strategies.
It is estimated that one third of the global population is infected with the tubercle bacillus. None of the current control measures consider the enormous latent reservoir of TB that keeps perpetuating the epidemic. Our goal is to identify biomarkers with prognostic potential, which will be crucial for designing and testing improved vaccines, which protect people with latent infection from developing TB disease.
Sophisticated molecular and immunological tools will be used to study (i) immune responses against the tubercle bacillus during natural infection in endemic populations in Africa, (ii) the impact of progressive HIV-1 infection and its treatment on immunity against TB as well as (iii) immune responses evoked by vaccination with BCG and novel TB vaccine candidates. This will enable the identification of biomarkers needed to facilitate and optimize the development of new TB vaccines.

Our Project: Biomarkers of Protective Immunity Against TB in the Context of HIV/AIDS in Africa
Our GCGH project falls under GOAL 2: To create new vaccines.
- The theme of Grand Challenge is: Learn which immunological responses provide protective immunity.
Principal Investigator: Stefan H.E. Kaufmann, Max Planck Institute for Infection Biology, Germany
Prof. Stefan H.E. Kaufmann heads an international consortium of 15 leading institutions across Europe, Africa, and the U.S. The focus of the study is to identify immune systematic differences between individuals who are exposed to tuberculosis and never become sick, and those who develop serious disease. The researchers are addressing the issue of TB in the context of HIV (people infected with both TB and HIV). The study results could help guide the design and testing of new intervention such as TB vaccines, drugs, and diagnostics, especially in areas with high HIV infection rates to combat this global public health problem.

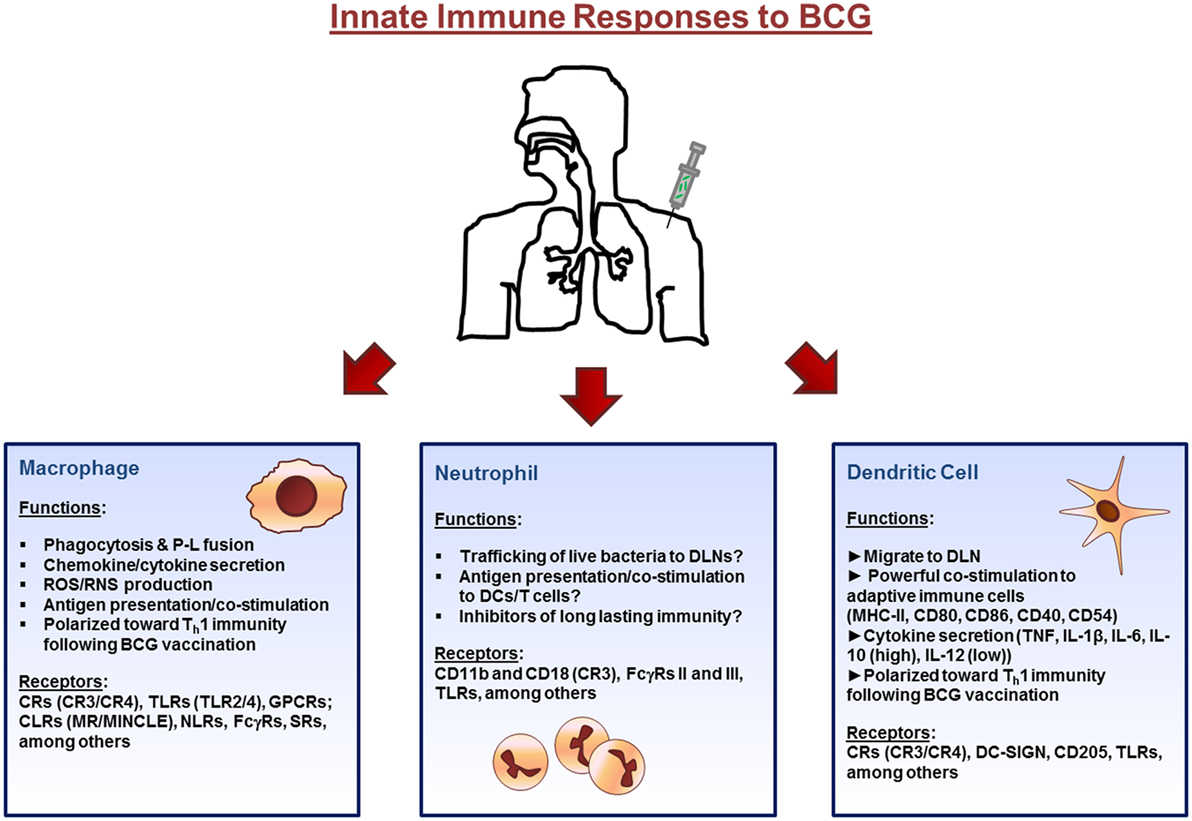
Roadblocks to progress
The major roadblock in the development of effective new TB vaccines is a lack of our understanding of what constitutes protective immunity during natural infection with Mtb. We expect that insight into the mechanisms of protection and TB disease progression will enable us to define immune correlates and host biomarkers of disease that can predict whether new TB vaccines will be effective. Biomarkers are also expected to play an important role as surrogate endpoint markers in large-scale field trials with new TB vaccines and hence will facilitate the iterative process of vaccine optimization during clinical trials.
Grand Challenge – Project GoalThe
Grand Challenge addressed is to learn which host responses provide protective immunity against TB. The goal of the project is to identify correlates of protective immunity and host biomarkers of TB disease with prognostic potential. These biomarkers will be essential decision support tools for the design and evaluation of novel and improved TB vaccines in both Africa and the rest of the world.
Work Packages
Six Major Activities or Workpackages (WP) will be covered, each with its specific focus and contribution to meet the selected Grand Challenge
Strategy And Specific Objectives
To address this Grand Challenge, sophisticated molecular and immunological tools will be applied by the participating laboratories in the USA and Europe to study host and pathogen responses to latent Mtb infection and active disease in individuals from 5 TB-endemic countries across the African continent. The participating researchers from developing countries and field sites are well established and provide excellent know-how and infrastructure to carry out clinical and natural history studies of TB and HIV-1 infection/AIDS, medical interventions and sophisticated laboratory studies. The Consortium combines multiple scientific disciplines: clinical sciences, epidemiology, microbiology, genomics, genetics, and molecular and cellular immunology. Six Major Activities or Workpackages (WP) will be covered, each with its specific focus and contribution to meet the selected Grand Challenge.
WP1: Profiling Of Pathogen Response – Identification Of Antigen-Specific T Cell Responses
We will perform genome-wide expression profiling of multiple field isolates of Mtb from the African continent using in vitro conditions that mimic different stages of persistent Mtb infection and disease, in addition to profiling of clinical specimens. This will provide a rational basis for selecting MTB antigens that are potentially targeted by the immune system. Subsequently, we will identify and characterize immune parameters of antigen-specific T cell responses for longitudinal studies in Africa.
WP2: Profiling Of Host Response To Infection – Identification Of Host Markers
We will perform genome-wide expression profiling of local and systemic host responses to Mtb infection and active TB disease using clinical specimens of TB patients, latently infected household contacts and controls. Candidate biomarkers will be verified by multiplex analyses and further analyzed for correlations with prevalent lymphocyte populations and functional properties to identify immune parameters for studies in the field.
WP3: Natural Protective Immunity Against TB
Development of natural protective immunity will be evaluated during follow up of household contacts with recent Mtb infection. We will recruit prospective cohorts of HIV-1 seronegative individuals at 4 African sites and follow them for 2 years to monitor TB disease progression. Longitudinal analyses will include antigen-specific T cell responses and expression of host biomarkers in order to define biomarkers of natural protective immunity and of TB disease.

WP4: Impact Of HIV-1 Infection/Aids And Response To Treatment
To assess the impact of progressive HIV-1 infection on immunity against Mtb, including the effect of antiretroviral treatment, we will recruit prospective cohorts of HIV-1 seropositive individuals at 5 African sites and prospectively follow them for 2 years to monitor acquisition or progression of Mtb infection. Patients on anti-retroviral therapy will be monitored for the development of immune reconstitution syndromes. Longitudinal analyses will be performed of antigen-specific T cell responses and expression of biomarkers in the context of HIV-1 infection in order to refine the set of biomarkers for TB.
WP5: Protective Immunity Following Vaccination With Bcg And Novel TB Vaccine Candidates
We will recruit and follow infants and adolescents who will be vaccinated with BCG. We will monitor the induction of immune responses against mycobacterial antigens and changes in expression levels of biomarkers and correlate these data with the level of protective immunity that is achieved by BCG. In addition, we will exploit the opportunity to test the biomarkers in interlocking studies with new TB vaccine candidates that are scheduled to take place in The Gambia, Ethiopia and South Africa.

WP6: Management And Coordination Of Project, Central Tasks And Reserves
We will ensure effective management of the project, including regular scientific exchange and financial reporting, management of Intellectual Property and ethical affairs. We will facilitate efficient communication and collaborations between the participating sites; foster capacity building, technology transfer and harmonization of clinical protocols, data management and laboratory assays.
Accomplishments
In the second year of the project, the GC#6-74 consortium is striding forward in achieving its objectives by recruiting subjects for longitudinal cohort studies and effectively establishing a functional cohesive multi-centered, multi-cultural team across the three continents.
- Screening of 86 novel recombinant antigens relevant to latent TB and reactivation TB have been completed in at least 20 tuberculin skin test (TST) positive healthy household contacts at each of the field sites.
- Final consensus made on the basis of the results in selecting first set of antigens for inclusion in the cohort studies across WP-3-4-5.
- Expression profiling from Mtb-infected lung tissues and peripheral blood mononuclear cells (PBMC) to select a set of host biomarkers
- Establishment of cohorts at field sites for immuno-profiling and host expression profiling through longitudinal studies over the two years follow-up period
- Establishment of HIV-positive cohorts at field sites and anti-retroviral therapy (ART) roll-out at most sites
- Completion of two year follow-up of infant cohorts for studies in BCG vaccinees
- Establishment of adolescent cohort in Cape Town


Environmental and Zoonotic TB Detection
- Emerging studies in environmental and zoonotic reservoirs of TB (e.g., in beagles or rodents) often rely on:
- Beagle plasma samples
- Environmental swabs using OmniSwab
- Selective media such as Ashby's mannitol agar and solid plates
- Some researchers investigate co-infections or immune cross-reactivity with other pathogens such as Salmonella typhi O, Salmonella paratyphi BH, or Typhi O, adding another layer of complexity to TB diagnostics.
Advanced TB Detection Reagents and Innovations
- Modern TB diagnostics also benefit from chemical tools and antibodies:
- 4G8 antibody, 6E10 antibody, and acetylated p53 antibody: Common in TB-associated neurodegeneration studies
- Dolichos biflorus lectin and sh-PEG-COOH: In nanocarrier-based biomarker delivery systems
- Cumate-inducible systems: Used in synthetic biology for TB antigen expression
- Hydrochloride-based solubilization and 10% sodium azide for preservation and reagent stabilization

Innovative Techniques and Consumables
- Phosphatase test in milk or phosphatase test for milk: Repurposed in TB for evaluating mycobacterial phosphatase activity
- Faramount: Used for immunofluorescence analysis
- Magenta boxes and incubator chambers ensure optimal sample incubation and storage
- Silwet L-77: A surfactant used in plant-based TB modeling studies
- Rare compounds like P7C3 for sale, robotnikinin, or flashBAC systems further expand therapeutic and vaccine research